
Happy birthday to my Foundational Lectures!
5-years ago today, I released the first of my nine foundational exosome videos.
I think they have aged like fine wine!

5-years ago today, I released the first of my nine foundational exosome videos.
I think they have aged like fine wine!
In the three years since I started using and learning about exosomes, they have become “FDA-approved’ (for Covid-19 mitigation). Let’s explore what that means
The exosome blog discusses a friend of mine whose nerve pain resolved after exosomes.
The telomere blog shows my theory of telomere erosion causing cancer and disease is valid
Dr. Ed Park of Recharge Biomedical tries to explain what Mesenchymal Stem Cells are and why their exosomes are so special

Dr. Ed Park of Recharge Biomedical addresses FAQs about exosome treatments. How are they different from stem cells? Are they FDA-approved? How much does it cost? What are his results?

Duncan Ross, founder of Kimera Labs, explains how he discovered the power of exosomes and how they will be FDA approved
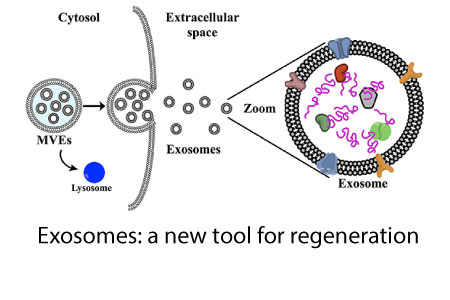
The FDA recently issued a warning about exosomes so we may have a window that will be closing. If you want to receive all the passwords and future blogs, all you need to do is contact me with an email to drpark@rechargebiomedical.com

DISCLAIMER: This website is for educational purposes only and is not for advertising. Telomerase activators and nanovesicles are not FDA-approved to prevent or treat any disease and anecdotes are not scientific proof of efficacy. All patients were treated in the context of a fully informed and legally-protected patient physician relationship.
© 2020 Recharge Biomedical
Exosomes and TA-65 are not FDA-approved to prevent or treat any illness